The BRIGHT project, funded by EIT Health, unveils a new model for breast cancer prevention based on genetic risk. This model implements clinical-grade polygenic risk score testing into healthcare and was presented at the European Society of Human Genetics Conference.
The BRIGHT project, led by scientists of the University of Tartu in collaboration with health technology company Antegenes, has presented pioneering research at the European Society of Human Genetics (ESHG) conference, highlighting the transformative potential of polygenic risk scores (PRS) in breast cancer personalized prevention. The project demonstrated the feasibility, acceptability, clinical utility and cost-effectiveness of genetics-based screening.
Breast cancer remains a leading cause of cancer-related deaths among women, and traditional screening methods overlook younger women with elevated risks. The BRIGHT project addressed this gap by integrating PRS and monogenic pathogenic variant (MPV) testing into the screening process. This approach allows for personalized prevention strategies, enabling earlier detection and intervention for high-risk individuals.
Key Findings from the BRIGHT Study
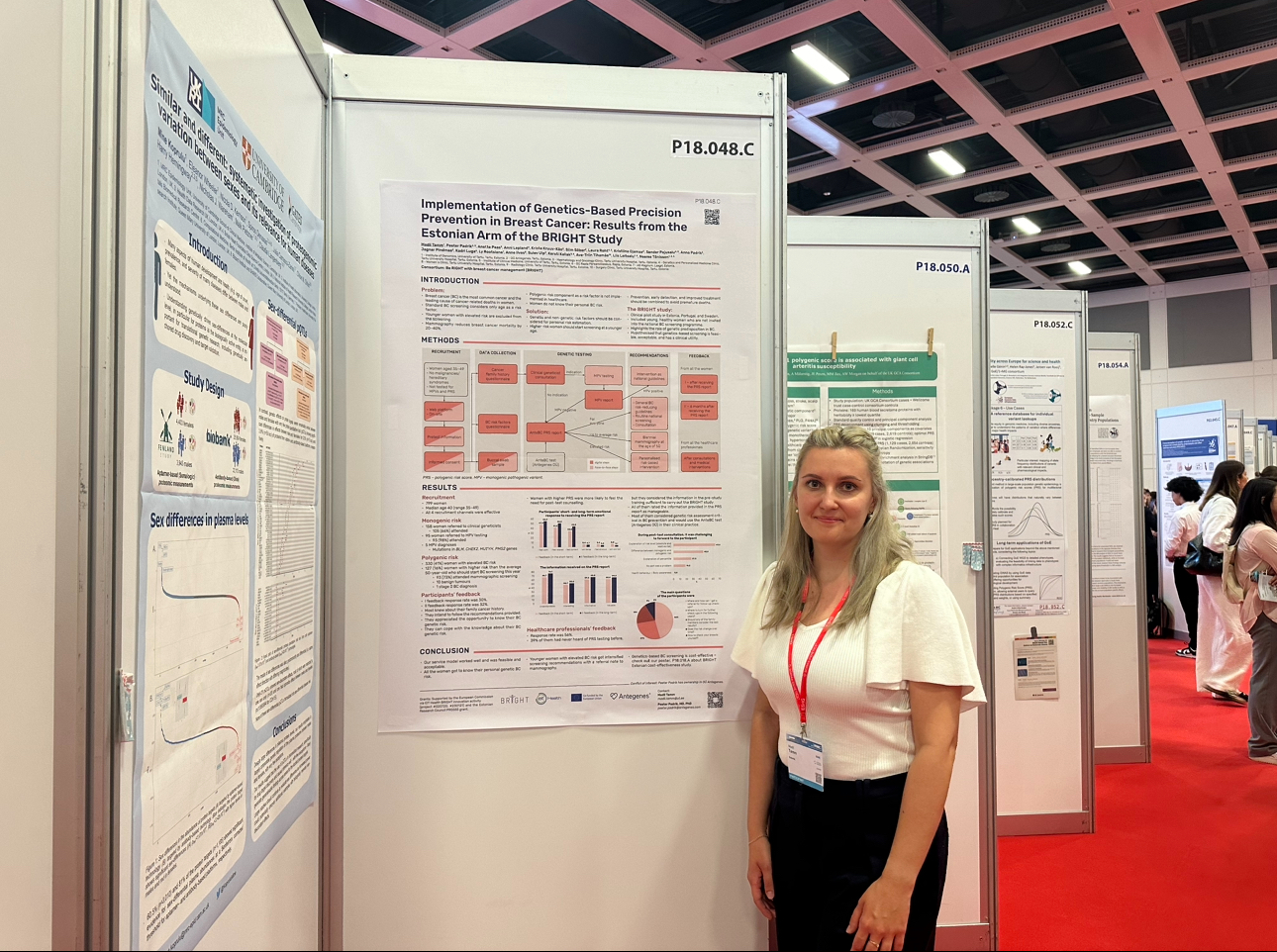
The Estonian arm of the BRIGHT study evaluated the polygenic risk of 799 women aged 35-49, with the aim to identify women with elevated risk to start their breast cancer screening earlier than the current standard from age 50. 330 (41.3%) participants were identified with elevated breast cancer risk through PRS testing. Notably, 127 (15.9%) women were found based on PRS to have a higher risk than the average 50-year-old already now, prompting earlier and more frequent screenings. They started with their personalized screening, resulting in one immediate stage 0 BC diagnosis. Additionally, the need for MPV testing was defined based on a family history of cancer according to guideline criteria. 93 women were tested with 5 MPV findings for intensified breast cancer prevention.
Cost-Effectiveness and Clinical Utility
A separate study within the BRIGHT project assessed the cost-effectiveness of PRS-based breast cancer screening in Estonia. Utilizing a Markov model, researchers compared the current age-based screening program with a PRS-tailored approach. The findings revealed that PRS-based risk-stratified screening resulted in more early-stage cancer detections and fewer advanced-stage cases and averted 15 breast cancer deaths per 10,000 women screened with an incremental cost-effectiveness ratio (ICER) of €37,755 per quality-adjusted life year (QALY) gained. This indicates that the PRS-based approach is not only clinically beneficial but also economically viable.
Positive Feedback and Future Implications
Feedback from both participants and healthcare professionals has been positive. Participants appreciated the opportunity to understand their genetic risk and were more likely to adhere to personalized prevention recommendations. Healthcare professionals recognized the value of integrating genetic risk assessments into routine practice, which could revolutionize breast cancer screening and reduce mortality rates.
The integration of breast cancer polygenic risk score testing and parallel monogenic variants management in healthcare aims to establish a stratified cancer screening approach that ensures better patient outcomes, improved equity and increased screening participation rates. Furthermore, the predominantly digital service could minimize the burden on healthcare personnel. A similar project is still being conducted in Portugal and Sweden.
About the BRIGHT project
This innovative approach is led by the BRIGHT consortium, which includes the University of Tartu, OÜ Antegenes, Tartu University Hospital, IESE Business School, GE Healthcare, Uppsala Region, Uppsala University Hospital, North Lisbon Central Hospital and Estonian Health Insurance Fund.
The BRIGHT project has been supported by the European Commission via EIT Health BRIGHT innovation activity (project #220720). For more information about the BRIGHT clinical study and breast cancer risk-based stratified screening service, please visit https://brightscreening.eu.
Presentations:
- Tamm M, Padrik P, Paas A, Lepland A, Kruuv-Käo K, Sõber S, et al. Implementation of Genetics-Based Precision Prevention in Breast Cancer: Results from the Estonian Arm of the BRIGHT Study. Poster P18.048.C. European Society of Human Genetics Conference; Berlin 2024.
- Sampaio F, Padrik P, Kruuv-Käo K, Lutsar K, Tõnisson N, Feldman I. Cost-Effectiveness of a Polygenic Risk Score Based Breast Cancer Screening Program in Estonia. Poster P18.018.A. European Society of Human Genetics Conference; Berlin 2024.